This site is intended for US
Healthcare Professionals only
This site is intended for US Healthcare Professionals only
Menu
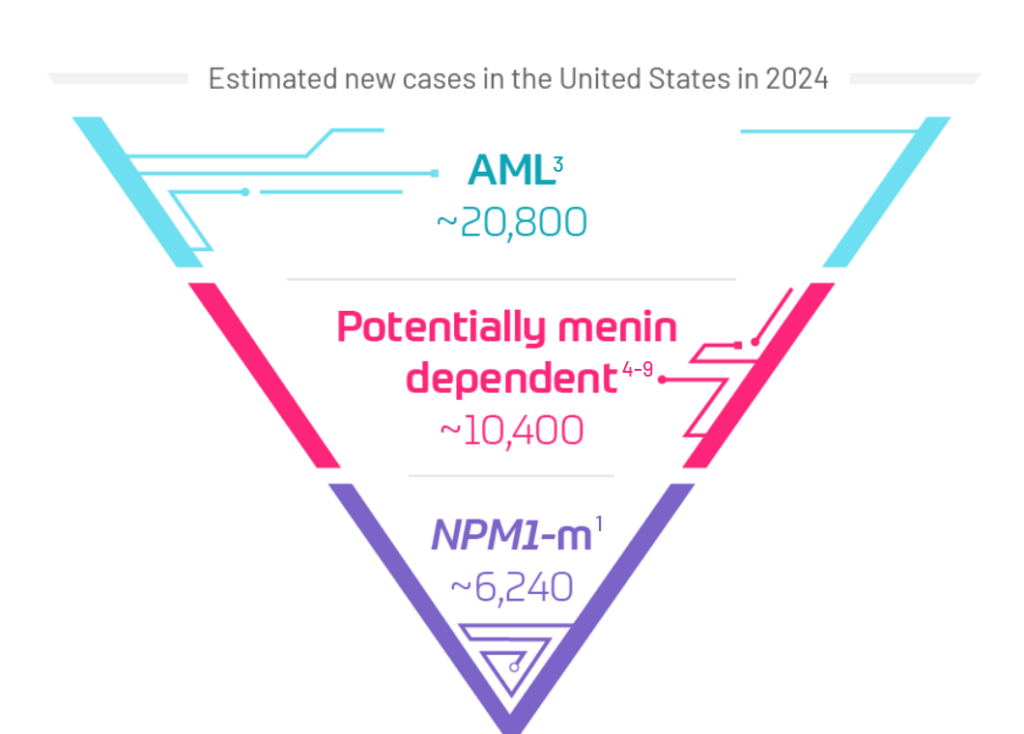
NPM1-m AML remains challenging, with considerable variability in patient outcomes across the treatment continuum.10-13
In patients with NPM1-m AML,
Similar to those in the relapse setting without NPM1m,
Patients with relapsing NPM1-m AML face low survival rates12
NPM1m
NPM1-wt
NPM1m
NPM1-wt
bBased on a retrospective analysis of patients with R/R AML with NPM1m (n=206) compared with NPM1-wt (n=1516)
treated with standard-of-care therapies.12
bBased on a retrospective analysis of patients
with R/R AML with NPM1m (n=206) compared
with NPM1-wt (n=1516) treated with standard-
of-care therapies.12
Current research exploring the potential of menin inhibition for menin-dependent AMLs, such as NPM1-m, may offer new hope for patients and the AML community.4,5
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend expedited testing for NPM1m at diagnosis and for repeat testing at relapse or progression to guide treatment.15
Detection of NPM1m drives to a critical diagnosis of AML, even at ≥10% myeloid blasts or equivalents,c regardless of prior history of MDS.1,16
Comprehensive mutational profiling, including NPM1m, is critical for prognosis, risk stratification, and guiding treatment decisions.15,16
As new research emerges, routine testing for NPM1m may enable tailored decision-making in the future.15,16
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; NCCN, National Comprehensive Cancer Network® (NCCN®); NPM1-m, mutated nucleophosmin 1; NPM1m, nucleophosmin 1 mutation; NPM1-wt, wild-type nucleophosmin 1; OS, overall survival; RFS, relapse-free survival; R/R, relapsed/refractory.
Sign up for clinical updates.
"*" indicates required fields